ABSTRACT In this study, the synthesis and characterization of poly(maleic anhydride- alt-1-octene) (poly(MA-alt-1-O)) were carried out. The syntheses were carried out in the form of free radical copolymerization at a constant temperature bath of 70 °C using benzoyl peroxide initiator and 1,4-dioxane solvent. Synthesis of the polymers were studied by using different monomer mole ratios. Copolymers were isolated from the reaction mixture by precipitation with methanol, then washed with several portions of 1,4-dioxane and dried at 40 °C under vacuum. For the characterization of the copolymer, first the viscosimetric measurements were carried out at 25 ±0.03 °C in water bath to investigate the relationship between the monomer concentrations and the molecular weights of the synthesized polymers. Then the structures of the synthesized copolymers were clarified by nuclear magnetic resonance (NMR), elemental analysis, titration and fourier transform infrared (FTIR) techniques. The reactivity ratios (r1 and r2) of the monomers used with respect to each other were determined using the data obtained from the FTIR method. The obtained monomer reactivity ratios showed that all copolymers were alternating copolymers (r1.r2 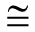 0). The composition of the synthesized copolymers was clarified by both elemental analysis and FTIR methods, and finally, their thermal behavior was studied by thermogravimetric analysis (TGA) method. |

